Introduction:
Paediatric Inflammatory Multisystem Syndrome - temporally associated with SARS-CoV2 (PIMS-TS) is a newly described syndrome during the COVID-19 pandemic. It is characterised by a state of persistent fever, inflammation and organ dysfunction following exposure to SARS-CoV-2 virus. It shares similar clinical features to Kawasaki disease, toxic shock syndrome and macrophage activation syndrome. Like adult COVID-19 infection, profound inflammatory response significantly increases the risk of thromboembolism, which is a major cause of morbidity and mortality. In this review we share our experience, observation and prevention strategy with regard to thromboembolism in PIMS-TS.
Method:
Between the period of 14th March 2020 to 31st May 2020, Evelina London Children's Hospital admitted 68 patients with PIMS-TS from the South Thames Network. Patients were broadly treated with a combination of high dose aspirin and immunomodulation in the initial period. Realising its pro-thrombotic potential, from 1st May 2020, treatment strategy was changed to incorporate prophylactic subcutaneous low molecular weight heparin (dalteparin 100units/kg once daily) in combination with low dose aspirin (5mg/kg once daily, maximum 75mg) when a working diagnosis of PIMS-TS was made. We retrospectively collected data to understand the prevalence and pattern of thromboembolism in this cohort of patients.
Results:
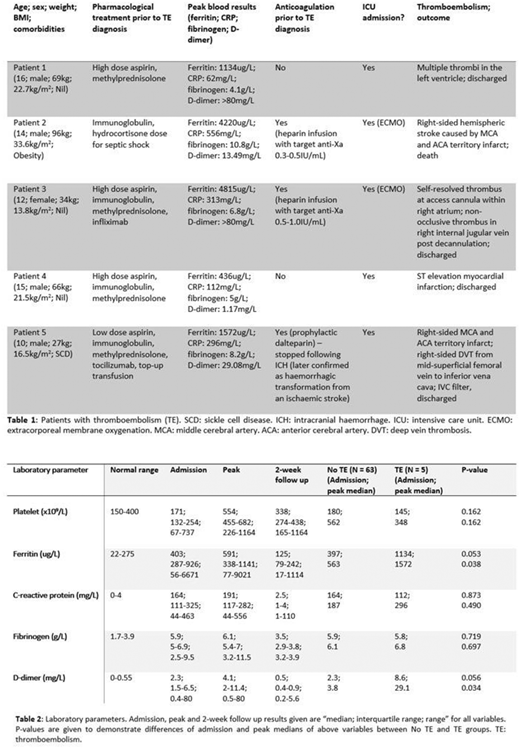
Five patients (7.4%) developed thromboembolic complications during their acute illness with 6 recorded events overall. (Table 1) Three events happened without prophylactic anticoagulation cover. Four (5.9%) were cardiovascular/arterial events and 2 (2.9%) were venous thromboembolism. Those who required ventilation support and extracorporeal membrane oxygenation (ECMO) were associated with higher thrombotic rate using 2-tailed Fisher's exact test (ventilation: p=0.02; ECMO: p=0.004). One ischaemic cerebrovascular event resulted in death. Two bleeding events were recorded whilst on anticoagulation. One was iatrogenic following arterial line insertion and another a haemorrhagic transformation from an ischaemic stroke.
Coagulation markers (fibrinogen and D-dimer) followed the overall pattern observed in inflammatory markers (ferritin and C-reactive protein) on admission, at peak values and at 2-week follow up, suggesting a connection between the degree of inflammation and hypercoagulability and inferring immunothrombosis. (Table 2) Peak ferritin and D-dimer levels were significantly higher in the group with thromboembolism using 2-tailed Mann-Whitney U test (ferritin: p=0.038; D-dimer: p=0.034).
Conclusion:
Thromboembolism needs to be considered as a significant complication in patients with a diagnosis of PIMS-TS. We observed more cardiovascular/arterial events than venous events, which coincided with the fact that PIMS-TS patients commonly develop cardiac involvement and coronary abnormalities. In addition to rapid correction of hyper-inflammatory state using immunomodulation, we suggest the consideration of low dose aspirin (5mg/kg once daily, maximum 75mg) and prophylactic dalteparin (100units/kg once daily) to prevent both arterial and venous thrombosis after careful individual assessment and exclusion of co-existing factors that can cause increased bleeding risk. This may be especially relevant in those with already raised ferritin and D-dimer levels on admission.
Inusa:Vertex: Research Funding; AstraZeneca: Honoraria, Other: Steering committee participation, Research Funding, Speakers Bureau; Bluebird bio: Research Funding; Novartis: Honoraria, Other: Steering committee participation, Research Funding, Speakers Bureau; Global Blood Therapeutics: Honoraria, Other: Steering committee participation, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal